FDA Proposes Front-of-Package Label for Food Products
At long last, the FDA unveiled its proposed Front-of-Package (FOP) nutrition information label (90 Fed. Reg. 5426).
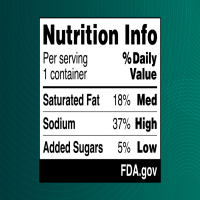
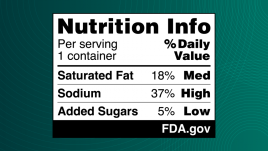
The proposed label – also called a Nutrition Info box – provides information on a product’s saturated fats, sodium and added sugars content and indicates whether the food has low, medium or high levels of those nutrients.
In a Jan. 14 press briefing on the proposal, Robin McKinnon, acting director of the Human Foods Program’s Nutrition Center of Excellence, said the high, medium and low were based on the long-standing research, which classifies 20 percent or more of the daily value as being high, 5 percent of the daily value or less as low and medium as being in between.
McKinnon noted the agency tested three types of FOP labels. “We found that the nutrition info scheme performed best overall in helping consumers identify healthier food options based on the levels of nutrients displayed for saturated fats, sodium and added sugars. Participants provided more correct answers regarding the healthfulness of product than the other schemes tested and also spent significantly less time evaluating the nutrient profile of the product.”
In addition, “of the five variations of the nutrition info scheme, the black and white version with the percent daily values performed the best in most cases,” she said. “For example, participants considered the black and white version with the percent daily values as the most useful, most trustworthy and the easiest to use. And all of the findings were consistent across demographic groups.”
The FDA noted its experimental study tested variations of the Nutrition Info box that included the colors green, yellow and red to help communicate to consumers that a product was low, medium, or high, respectively, in the listed nutrients to limit. “While there is some information in our literature review suggesting that color coding with text can lead to improved understanding of nutrition information, our experimental study found that colors neither significantly increased the utility of the Nutrition Info box for U.S. consumers nor increased understanding of the interpretive information provided in a statistically significant way,” the notice said. “Additionally, we have heard concerns from interested parties about potential difficulty with using color coding for consumers with red-green color blindness, which is the most common form, and that mandating colors would substantially increase the cost of complying with the rule. Given these factors, the importance of maintaining consistency for consumers, and to facilitate comparisons between products, our proposal would not require or allow such colors.”
“Since the Nutrition Facts label was last updated in May 2016, FDA has tentatively determined that additional, interpretive nutrition information on the front of food packages – to provide context for certain nutrient declarations – is necessary to help consumers more easily observe and better understand and use this information when building their diets,” the FDA announcement of the proposed rule said.
“The science on saturated fat, sodium and added sugars is clear,” FDA Commissioner Robert Califf, said in announcing the proposed rule. “Nearly everyone knows or cares for someone with a chronic disease that is due, in part, to the food we eat. It is time we make it easier for consumers to glance, grab and go. Adding front-of-package nutrition labeling to most packaged foods would do that. We are fully committed to pulling all the levers available to the FDA to make nutrition information readily accessible as part of our efforts to promote public health.”
“Requiring the Nutrition Info box would provide consumers with standardized and factual context about important nutrients on the front of food packages that can be compared across products,” the FDA notice said. “To streamline the amount of information provided in, as well as the space we require manufacturers to use for, the proposed Nutrition Info box, we are focusing on the three nutrients to limit to ensure that consumers have this additional context about these three important nutrients on the front of food packages to help inform their food choices.”
The proposed rule, if finalized, would require food manufacturers to add a Nutrition Info box to most packaged food products three years after the final rule’s effective date for businesses with $10 million or more in annual food sales and four years after the final rule’s effective date for businesses with less than $10 million in annual food sales.
The FDA also is proposing to amend 21 C.F.R. § 101.61(b)(4) and (5) and 21 C.F.R. §101.62(c)(2) and (3) to specify that a food subject to this rule must display “Low” in accordance with 21 C.F.R. §101.6 for sodium or saturated fat in the Nutrition Info box to qualify for a low sodium or low saturated fat nutrient content claim, respectively. “A food bearing a low sodium or low saturated fat nutrient content claim but falling into the ‘Med’ or ‘High’ categorization for that nutrient in the Nutrition Info box would lead to inconsistency in the labeling of such food and could result in consumer confusion,” the FDA said. “Therefore, we tentatively conclude that a food subject to this rule must display ‘Low’ in accordance with 21 C.F.R. §101.6 for the respective nutrient in the Nutrition Info box to qualify for a low sodium or low saturated fat nutrient content claim. In addition to updating the claim to reflect current nutrition science and helping to avoid consumer confusion by aligning with the Nutrition Info box’s ‘Low’ description, this amendment would also address products that are not subject to the proposed requirement to display a Nutrition Info box,” the FDA said.
The FDA proposed revising the definitions for the low sodium nutrient content claim and low saturated fat nutrient content claim to require that, in order to bear a low sodium nutrient content claim or low saturated fat nutrient content claim, a food must display “Low” in accordance with proposed 21 C.F.R. §101.6 for sodium or saturated fat in the Nutrition Info box, respectively. The agency noted it was not proposing to update the gram amount for the low saturated fat nutrient content claim, as the gram amount for the low saturated fat nutrient content claim (1 g saturated fat) already aligns with the proposed definition for the “Low” interpretive description in the Nutrition Info box (at 5 percent DV or less).
The FDA also ruled the proposed required information in the Nutrition Info box does not constitute a nutrient content claim and is not subject to the requirements for nutrient content claims. Thus, the agency proposed amending 21 C.F.R. §101.13(c) to specify that the information proposed to be required as part of the Nutrition Info box would not be a nutrient content claim. “This is consistent with our determination that the information in the Nutrition Facts label, including percent DV, is not a nutrient content claim,” the agency said, noting “any claims about the nutrient levels of a food outside of the Nutrition Facts label and proposed Nutrition Info box would be nutrient content claims, which must meet applicable statutory and regulatory requirements and the relevant nutrient content claim regulations.”
The proposed rule also exempts most dietary supplements from bearing a Nutrition Info box. “While dietary supplements are considered foods, they serve a different purpose than conventional foods when it comes to building a healthy dietary pattern; they often provide individual nutrients (e.g., Vitamin C) and are intended to supplement, rather than constitute a core part or foundation of, the diet,” the notice said. “Many dietary supplements do not contain saturated fat, sodium, and added sugars, and they are not required to have these nutrients declared on their nutrition label unless the supplement contains quantitative amounts by weight that exceed the amount that can be declared as zero (see 21 C.F.R. §101.36(b)(2)). However, we are aware that some dietary supplements may contain what this proposed rule would describe as ‘High’ levels of saturated fat, sodium, or added sugars per serving. We therefore invite comment on our proposed exemption of dietary supplements from the requirements of this rule.”
Proposed 21 C.F.R. §101.6(a)(1) would require that the rule apply to all food covered under 21 C.F.R. §101.9 (nutrition labeling of food) that is marketed for people ages 4 and older unless a specific exemption applies. “We recognize that infants and children ages 1 to 3 years are vulnerable subpopulations and have specific nutritional needs,” the notice said, adding the “FDA has not established DRVs or percent DVs (i.e., how much a nutrient in a single serving of food contributes to the DRV) for saturated fat, sodium, or added sugars for infants through age 12 months.”
The notice requested comments, including data and other information, related to the nutritional needs of infants and young children and the need for or value of interpretive nutrition information that can help consumers quickly and easily identify how foods can be part of a healthy diet for them. “We expect such feedback could help inform any future FOP policy for foods marketed for infants and children ages 1 to 3 years,” the FDA said.
Proposed 21 C.F.R. §101.6(a)(2)(i) would require using “Nutrition Info” as the heading, or title, for the Nutrition Info box. The title describes what the box conveys and should be familiar in appearance to the “Nutrition Facts” title of the Nutrition Facts label (21 C.F.R. §101.9(d)(2)). “We are proposing ‘Info’ rather than ‘Information’ to keep the title shorter and, therefore, the box smaller. This title reflects our intent to provide, in a convenient format, interpretive nutrition information to consumers that can help them quickly and easily identify how foods can be part of a healthy diet,” the notice said.
The title would be displayed across the same distance of the box as the “Nutrition Info” header to orient the consumer and make it clear that the nutrition information that follows is part of the Nutrition Info box. “For the Nutrition Info box to be useful, consumers need to understand what nutrition information is being conveyed,” the FDA said.
Other Proposed Provisions Detailed
Proposed 21 C.F.R. §101.6(a)(2)(ii)(A) would require using the subheading “Per serving” to help consumers understand that the Nutrition Info box, like the Nutrition Facts label, provides information about one serving of the food. The FDA also proposes including a statement of the serving size, expressed in household measures, alongside the “Per serving” subheading (e.g., “Per serving (whole package)” or “Per serving (1/2 cup)”) to “further help consumers understand what one serving is. The inclusion of both ‘Per serving’ and the serving size expressed in household measures,” the agency said, “would help consumers understand whether the product is ‘Low,’ ‘Med,’ or ‘High’ in the three nutrients disclosed for a specific amount of the product.” In addition, “listing ‘Per serving’ with a statement of the serving size expressed in household measures would help improve awareness that the information presented in the Nutrition Info box does not refer to the contents of the entire package when the package contains multiple servings,” the FDA said.
Proposed 21 C.F.R. §101.6(a)(2)(ii)(B) would require including the subheading “% Daily Value” in the Nutrition Info box. The heading would appear above the declaration of the quantitative percent DV and the interpretive “Low,” “Med,” and “High” descriptions for the three nutrients included. The FDA proposed using the “%” symbol instead of the word “percent” due to spacing considerations and for consistency with the Nutrition Facts label.
Proposed 21 C.F.R. §101.6(a)(2)(iii)) would require that the Nutrition Info box include information on saturated fat, sodium, and added sugars. “We propose that saturated fat, sodium, and added sugars be the only nutrients in the Nutrition Info box, given their significance in building healthy dietary patterns, the scientific research on FOP nutrition labeling, and the current food labeling landscape, in which consumers report being familiar with a wide variety of industry claims.” The notice said “manufacturers have many ways to communicate information on the front of a food package about nutrients to get enough of. Use of nutrient content claims can inform consumers interested in intake of specific nutrients.”
The FDA considered including a calorie disclosure in the proposed Nutrition Info box. The agency concluded “a quantitative calorie statement would not provide consumers with new, interpretive information.” In addition, “While these DRVs are based on the reference caloric intake of 2,000 calories, which we use for general nutrition advice, we note that there is no DRV, and therefore no percent DV, for calories.”
The FDA invited comments on the inclusion of a mandatory or voluntary quantitative statement of calories in the Nutrition Info box, as well as on ways the agency could consider inclusion of an interpretation of quantitative calorie information in the Nutrition Info box, including any new data or other information on which to base such an interpretation.
Proposed 21 C.F.R. §101.6(a)(2)(iv)) would require low, medium and high descriptions for each nutrient to limit in the Nutrition Info box. The FDA proposed a range of 5 percent DV or less for “Low”; 6 to 19 percent DV for “Med”; and 20 percent DV or more for “High.” In proposing the ranges, the FDA “considered the regulatory history related to establishment of the percent DV and such descriptions; our longstanding consumer education activities designed to help consumers understand the percent DV in the context of the total daily diet; the nutrition education initiatives of other groups; and our existing regulatory definitions for nutrient content claims, including definitions established for ‘low’ and ‘high’ claims.”
The FDA invited comment on serving size as well as data and other information on possible different approaches. Additionally, the agency requested data and other information on any alternative criteria for the proposed interpretive “Low,” “Med,” and “High” descriptions that could support the agency’s goals of providing consumers with interpretive information for the levels of the three nutrients to limit in the Nutrition Info box that can help them quickly and easily identify how foods can be part of a healthy diet. The FDA also wants comment on use of the “Low” categorization for products that declare 0% DV for any of the three nutrients, rather than a fourth categorization, such as “Zero” or “Free.”
Proposed 21 C.F.R. §101.6(a)(2)(v) would require that the Nutrition Info box include the corresponding quantitative percent DV for people ages 4 and older for each nutrient listed, which will have already been calculated for the Nutrition Facts label and have the values placed in a column underneath the “% Daily Value” subheading and to the left of the interpretive descriptions.
Proposed 21 C.F.R. §101.6(a)(2)(vi) would require the inclusion of a banner at the bottom of the Nutrition Info box that includes an “FDA.gov” attribution statement. “Several studies on FOP nutrition labeling found that inclusion of an attribution for the FOP nutrition label increases consumers’ trust in and the credibility of the information,” the FDA said. “We propose to include the attribution statement ‘FDA.gov’ in the Nutrition Info box to indicate that the proposed Nutrition Info box appears as required by FDA and to signal to consumers where they can find additional information regarding the Nutrition Info box, nutrition information, and our nutrition labeling requirements.”
Proposed 21 C.F.R. §101.6(a)(3) would require nutrition information to be presented on food labels or labeling in a specific format for the standard Nutrition Info box. The proposed Nutrition Info box’s format elements include type style (i.e., a single font); size (i.e., point); color; justification (i.e., left, right, or centered); and use of boldface and hairlines. “Use of a simple format aligns with our goal for FOP nutrition labeling, which is to provide consumers, including those who have lower nutrition knowledge, with interpretive nutrition information that can help them quickly and easily identify how foods can be part of a healthy diet. Thus, our proposed Nutrition Info box is designed to serve as a visual cue to the reader that the information it contains is quickly and easily understandable interpretive nutrition information about the three nutrients to limit. Additionally, while we are committed to the flexible application of graphic techniques to make the required nutrition information easy to read and comprehend, we want to ensure that all Nutrition Info boxes, regardless of the products they reflect, look similar so that consumers will immediately recognize them and understand what they are.”
Proposed 21 C.F.R. §101.6(a)(3)(i) would require the Nutrition Info box to appear on the upper third of the principal display panel. The FDA noted four consumer research studies on FOP nutrition label placement “found that consumers had improved attention, reaction time, and label understanding when the FOP nutrition label was in the upper left or right of the principal display panel compared to the lower left or right. However, we are aware that foods’ principal display panels often contain other informational and graphic design elements in addition to the information we require and that certain foods come in packages of different shapes. We want to balance our goal of providing consumers with interpretive nutrition information that can help them quickly and easily identify how foods can be part of a healthy diet with maintaining flexibility for industry in the design of their principal display panels.”
The FDA invited comment, including studies or other research, on the location and specifically on whether to take a flexible approach rather than designating the proposed Nutrition Info box’s exact location.
Proposed 21 C.F.R. §101.6(a)(3)(ii) would require use of a single, easy-to-read type style in the Nutrition Info box. The FDA used Helvetica in its example proposed Nutrition Info box, which is the same type of style used in the agency’s example Nutrition Facts label.
Proposed 21 C.F.R. §101.6(a)(3)(iii) would require the use of a minimum type size (at least 8 point) in the Nutrition Info box that is no smaller than the size of the required net quantity of contents declaration specified in 21 C.F.R. §101.7(h) and (i). Those regulations prescribe the following size specifications for net quantity declarations:
- Not less than one-sixteenth inch in height on packages the principal display panel of which has an area of 5 square inches or less;
- Not less than one-eighth inch in height on packages the principal display panel of which has an area of more than 5 but not more than 25 square inches;
- Not less than three-sixteenths inch in height on packages the principal display panel of which has an area of more than 25 but not more than 100 square inches; and
- Not less than one-fourth inch in height on packages the principal display panel of which has an area of more than 100 square inches, except not less than ½ inch in height if the area is more than 400 square inches.
If the declaration is blown, embossed, or molded on a glass or plastic surface rather than by printing, typing or coloring, then the lettering sizes are to be increased by one-sixteenth of an inch (21 C.F.R. §101.7(i)).
“We are also proposing an absolute minimum type size of 8 point, regardless of the size of the net quantity of contents statement,” the FDA said. “It is our tentative view that this is the minimum type size necessary to allow for quick and easy readability of the Nutrition Info box and for the Nutrition Info box’s information to be read and understood by the ordinary individual under customary conditions of purchase and use.”
Proposed 21 C.F.R. §101.6(a)(3)(iv) would require the use of one color (e.g., black) for all type and hairlines in the Nutrition Info box, and proposed 21 C.F.R. §101.6(a)(3)(v) would require the use of a neutral contrasting background color (e.g., white) to the print in the box. “Contrast levels between text and background that exceed 70 percent, which we would expect from the use of a single type of color and a neutral contrasting background color, and dark text on light backgrounds provide for optimal legibility,” the FDA said.
Proposed 21 C.F.R. §101.6(a)(3)(xv) would require the attribution banner background be the same color as used for the rest of the box’s type and hairlines and that the “FDA.gov” statement be the same color as used for the rest of the box’s background. “We tentatively conclude that the use of an opposite color scheme to the rest of the box would help visually differentiate the attribution banner from the food’s nutrition information. The attribution banner would appear effectively the same on every Nutrition Info box—it would be at the bottom of the box, in an opposite color scheme, and would always contain ‘FDA.gov’ right-justified in the banner. That would provide consistency among all Nutrition Info boxes and signal that the Nutrition Info box is an FDA requirement, while not drawing attention away from the nutrition information above it,” the FDA said.
The FDA added the proposal will allow flexibility for industry to choose background and type colors that meet the requirements in the regulations. “However, we believe that some contrasting color combinations might render the proposed Nutrition Info box’s text difficult to read. We would consider difficult-to-read type to be unclear, and therefore violative of proposed 21 C.F.R. §101.6(a)(3)(ii)’s requirement that type be clear and easy to read, which could potentially cause the product to be misbranded.”
Proposed 21 C.F.R. §101.6(a)(3)(vi) would require the use of hairlines to delineate the outer box, while proposed (vii) and (viii) would require the use of hairlines to distinguish information within the Nutrition Info box. “Horizontal lines are used throughout the Nutrition Facts label as a key graphic element to divide space, direct the eye, and similarly give the label a unique and identifiable look. We propose using hairlines in the same way for the Nutrition Info box.”
Proposed 21 C.F.R. §101.6(a)(3)(ix), (xi), and (xiii) would require the use of extra-bold type print and proposed 21 C.F.R. §101.6(a)(3)(xii) would require the use of bold type print to highlight certain text. “Bold or extra bold type print for certain Nutrition Info box elements would help consumers notice and locate the box and use the information the box contains,” the FDA said. “We propose the use of bold or extra-bold type print for all type in the box other than the ‘Per serving’ subheading, the household measurement declaration, and the quantitative percent DV. An extra-bold ‘Nutrition Info’ heading would call attention to the box itself and an extra-bold ‘% Daily Value’ subheading and the interpretive descriptions underneath the subheading would call attention to the nutrition information the Nutrition Info box would provide. Additionally, bold nutrient names would call attention to each nutrient separately.”
Proposed 21 C.F.R. §101.6(a)(3)(x) and (xii) would require left justification for the “Per serving” subheading and the nutrient names, respectively. Proposed 21 C.F.R. §101.6(a)(3)(xi) and (xiii) would require right justification for the “% Daily Value” subheading and the interpretive descriptions, respectively, and proposed 21 C.F.R. §101.6(a)(3)(xv) would require right justification for “FDA.gov” in the bottom banner. Proposed 21 C.F.R. §101.6(a)(3)(vi) would require center justification for the “Nutrition Info” heading. Proposed 21 C.F.R. §101.6(a)(3)(xiv) would require that the quantitative percent DVs be right justified with each other, in a column to the left of the interpretive descriptions. “This design would create white spaces in the box, which would help isolate elements of the Nutrition Info box and provide information pacing for the reader,” the FDA said.
Proposed 21 C.F.R. §101.6(a)(4) would prohibit any information in the Nutrition Info box other than what proposed 21 C.F.R. §101.6 would require. “Our research has found that too much information can be confusing to consumers. Additionally, our focus with the Nutrition Info box is to provide consumers with standardized, interpretive nutrition information that can help them quickly and easily identify how foods can be part of a healthy diet and allow them to compare nutrition information across foods,” the FDA said.
Proposed 21 C.F.R. §101.6(b)(1) would require packages that use an aggregate display for the Nutrition Facts label to display a Nutrition Info box for each different product the package contains or could contain. “While we are aware that the required display of multiple individual Nutrition Info boxes may occupy more space than an aggregate display, we reiterate that our public health goal with the Nutrition Info box is to provide consumers, including those who have lower nutrition knowledge, with interpretive nutrition information, at the point of decision-making, that can help them quickly and easily identify how foods can be part of a healthy diet,” the FDA said. “Separate, rather than aggregate, Nutrition Info boxes would better help consumers to quickly view this information since consumers would not need to refer back to nutrient names, among other information, to understand the interpretive information in the box. We are also concerned that an aggregate Nutrition Info box would appear cluttered and unclear, because we designed the proposed box to occupy as little space on the label as possible while still giving consumers information, including the interpretive high, medium, and low categorizations that can help them quickly and easily identify how foods can be part of a healthy diet.”
Proposed 21 C.F.R. §101.6(b)(1)(i) would require the Nutrition Info boxes to appear together in either horizontal or vertical lines. “This would mean that all Nutrition Info boxes on packages that may use an aggregate display for the Nutrition Facts label would appear on the label in an unbroken line or set of lines that run either vertically or horizontally. Grouping the boxes consistently and together on the package, rather than allowing them to appear in different locations on a label or labeling, would help consumers more easily find the boxes and reduce the likelihood that they might not see one of the boxes because it appeared in a different location.”
Proposed 21 C.F.R. §101.6(b)(1)(ii) would require each individual food’s name to appear right justified at the top of the food’s Nutrition Info box, separated from the “Nutrition Info” header by a horizontal, centered hairline rule. “Inclusion of each individual food’s name at the top of its Nutrition Info box would clarify which individual food’s nutrition information is represented by which Nutrition Info box,” the FDA said. “Right-justification of each individual food’s name would provide an alignment cue and create a sense of unity and cohesion, contributing to the box’s overall aesthetic and perceived stability. Use of a horizontal hairline to separate the individual food’s name from the ‘Nutrition Info’ header would divide space and give the box an identifiable look similar to that of the Nutrition Facts label.”
Proposed 21 C.F.R. §101.6(b)(2) would require the Nutrition Info box for products that present a dual-column Nutrition Facts label for multiple age groups to reflect only the nutrition information for people ages 4 and older.
Proposed 21 C.F.R. §101.6(b)(3) would require the Nutrition Info box for products that present a dual-column Nutrition Facts label for “per serving” and “per individual unit” nutrition information to reflect only the nutrition information “per serving.” “This is consistent with how the standard Nutrition Info box reflects nutrition information ‘per serving’ when the individual unit contains more than one serving,” the FDA said. “Inclusion of the required ‘Per serving’ subheading with a statement of the serving size expressed in household measure would inform consumers that the package contains more than a single serving.”
Proposed 21 C.F.R. §101.6(b)(4) would require that the Nutrition Info box for products that present a dual-column Nutrition Facts label for multiple forms of the same food and for common combinations of food reflect the food “as packaged.” The FDA also proposed that a statement clarifying that the box represents “as packaged” nutrition information appear right justified at the top of the Nutrition Info box and separated from the “Nutrition Info” header by a horizontal, centered hairline rule, similar to aggregate display information. “This would help ensure that consumers know the Nutrition Info box always represents the food as it is at the point of decision-making, without any additional ingredients that would change the percent DV or interpretive descriptions,” the FDA said.
Proposed 21 C.F.R. §101.6(b)(5) would allow foods in packages with a total surface area available to bear labeling of 40 or fewer square inches to use an alternative Nutrition Info box (intermediate-package Nutrition Info box). This box would be smaller than the proposed Nutrition Info box and is designed to balance the agency’s public health goal of providing consumers with interpretive nutrition information that can help them quickly and easily identify how foods can be part of a healthy diet with the reduced amount of space available to bear labels on intermediate-sized packages relative to larger packages. “Providing flexibility for these intermediate-sized packages to display a modified Nutrition Info box would be consistent with our Nutrition Facts label regulations,” the FDA said.
Proposed 21 C.F.R. §101.6(b)(5)(i) would establish the intermediate-package Nutrition Info box, which would omit the “Per serving” and “% Daily Value” subheadings and the agency “recognize[d] that consumers could use the percent DV declarations to, among other things, quickly compare products that have the same interpretive description for a given nutrient. For example, two products may both have ‘high’ added sugars interpretive descriptions, but one may contain 30% DV added sugars, while the other contains 60% DV added sugars. We also recognize that the quantitative percent DV, in addition to the interpretive descriptions, may help consumers better understand why a serving of a food has the interpretive description it does.”
The FDA wants comments on the exclusion of the quantitative percent DV and on other design factors or choices the agency could make to balance its public health mission and FOP nutrition labeling goals with the space constraints on intermediate-sized packages.
Proposed 21 C.F.R. §101.6(b)(5)(ii) would require the use of abbreviations for saturated fat (“Sat. Fat”) and added sugars (“Add. Sugar”) to help with potential overcrowding issues in the intermediate-package Nutrition Info box and to help ensure readability.
Proposed 21 C.F.R. §101.6(b)(6) would require that the labeling of foods sold to consumers from bulk containers display the Nutrition Info box plainly in view of the consumer at the point of purchase. “This presentation is the best way to ensure that consumers would have immediate access to that information at the point of decision-making—the same way they would have that information on the principal display panel of most packaged foods.”
Proposed 21 C.F.R. §101.6(b)(7)(i) would require game meats to display a Nutrition Info box that reflects how the nutrition information is presented under 21 C.F.R. §101.9. “If the Nutrition Facts label for a game meat presents nutrition information as packaged as required under 21 C.F.R. §101.9(b)(7), then the Nutrition Info box would reflect the nutrition information as packaged,” the FDA said. “However, if the Nutrition Facts label presents nutrition information as prepared, following the special labeling provision at 21 C.F.R. §101.9(j)(11), then the Nutrition Info box also would reflect the nutrition information as prepared. We are proposing this because the percent DVs used to determine the interpretive descriptions for each nutrient in the Nutrition Info box are based on a serving’s amount, in grams, of each nutrient. The values may differ between the nutrition information presented as packaged versus as prepared.”
Proposed 21 C.F.R. §101.6(b)(7)(ii) would require these game meats to display the Nutrition Info box plainly in view of the consumer at the point of purchase. “Providing such information clearly in view of the consumer on the labeling of game meat not in packages is the best way to ensure that consumers would have immediate access to that information at the point of decision-making,” the FDA said.
Proposed 21 C.F.R. §101.6(c)(1) would exempt certain foods, such as raw fruits and vegetables, from bearing a Nutrition Facts label.
Proposed 21 C.F.R. §101.6(c)(2) would exempt foods in small packages that have a total surface area available to bear labeling of less than 12 square inches from the requirement to display the Nutrition Info box. “While such a small package must bear the appropriately sized Nutrition Facts label if its label includes any other nutrition information, we tentatively conclude that there would not also be enough room to fit a Nutrition Info box on its label that would be legible to consumers without occupying much of the available space to bear labeling,” the FDA said.
Proposed 21 C.F.R. §101.6(c)(3) would exempt the outer packaging of gift packages from the requirement to display Nutrition Info boxes. “We tentatively conclude that the point of decision-making for gift packages is generally after the package is opened, when a consumer is considering which food from the gift package to eat. While we propose that the outer wrapping be exempt, we note that the inner food products would be subject to the requirements in this rule, unless otherwise exempted,” the FDA said.
Proposed 21 C.F.R. §101.6(c)(4) would exempt unit containers in a multiunit retail food package from FOP nutrition labeling, so long as the unit containers fall under the exemption for Nutrition Facts labeling in accordance with 21 C.F.R. §101.9(j)(15); and the multiunit retail food package bears the Nutrition Info box in accordance with 21 C.F.R. §101.6.
The FDA also is proposing to revise 21 C.F.R. §101.61(b)(4)(i)(A) and (B) so that a food other than a meal product or main dish product may bear a low sodium nutrient content claim if a serving of the food contains 115 mg or less sodium per RACC rather than 140 mg or less sodium per RACC; and 21 C.F.R. §101.61(b)(5)(i) so that meal products and main dish products may bear a low sodium nutrient content claim if a serving of the food contains 115 mg or less sodium per 100 g rather than 140 mg or less sodium per 100 g.
“This revision is consistent with the updated DRV for sodium in the 2016 Nutrition Facts label final rule and with FDA’s ongoing sodium reduction efforts,” the FDA said. “It also generally aligns with the 5% DV or less range that we are proposing for ‘Low’ for sodium in the proposed Nutrition Info box.”
Comments on the proposed rule can be submitted electronically at http://www.regulations.gov by May 16, 2025, to Docket No. FDA-2024-N-2910.